
Over a century ago, food preservatives were simple—pickling with vinegar, packing in salt, maybe a smoky fire for good measure. Advances in chemistry opened new doors in the 1900s, as scientists started blending acids to improve food storage. That’s where sodium diacetate found a home. Truth is, its roots trace back to the need for safer, pretty straightforward preservation—vinegar flavor, minus the mess and bite of liquid acid. By the mid-20th century, food manufacturers started to bank on its balanced sour-salty profile, especially in baked goods and snacks. In those early days, it cut down spoilage and gave foods like bread and chips an extra toastiness, steering clear of the mold and staleness so common before.
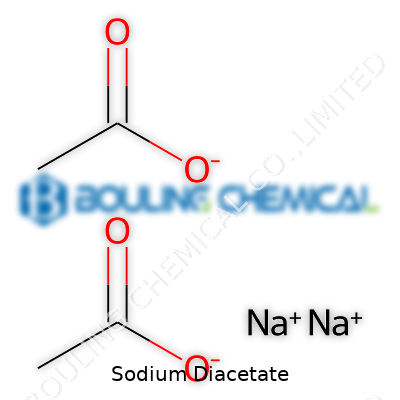
Sodium diacetate stands as a dry, white, free-flowing powder. It comes blended straight from acetic acid and sodium acetate. The product breaks down into a ratio roughly one part sodium acetate and one part acetic acid. Instead of wrestling with sloshing liquid vinegar, producers add a spoonful of sodium diacetate, which does the job without sogging up the batch. This formula, marked by a crisp sour tang, gets sprinkled on potato chips, cereals, tortillas, baked crackers, and sometimes in applications beyond food, like controlling odors or inhibiting microbial growth in animal feeds.
Sodium diacetate doesn’t hide its personality. It releases a sharp, acetic acid aroma as soon as you pop the lid. Under typical kitchen or warehouse conditions, it stays dry, clump-resistant, and crystal-like. Its solubility in water runs high, letting it dissolve with ease—no stubborn chunks or residue. Melting shows up around 150°C, at which point decomposition kicks off, driving acetic acid vapors into the air. Chemically, the pH runs acidic but not harsh, which is a big reason it works so well when tossed onto breads or snacks—enough zip to keep spoilage in check but not so much to burn the tongue.
As far as quality control goes, sodium diacetate generally clocks in at 58-60% acetic acid by weight. The industry keeps tight watch on color, particle size, and purity, because just a bit of extra moisture could mean caking, and too much dust, well, nobody likes clouds on a food line. Lab testing keeps microbial counts low, heavy metals well beneath safe thresholds, and assures it matches legal purity standards in major markets like the U.S., EU, and China. When folks see E262(ii) or a detailed spec sheet, it’s sodium diacetate lined up against expected chemical specs, with not much room for error—especially since regulators watch food additives like hawks.
Chemists prep sodium diacetate by combining pure, glacial acetic acid with sodium carbonate or sodium hydroxide in a specific ratio. The reaction heats up, water’s released, and a dry, salty-acidic product forms. Efficient facilities dry the solid out, grind it to fine powder, and run it through screens. The final product finds its way into moisture-proof, sealed bags to stop the powder from clumping up before it reaches bakers, chip makers, or industrial clients. Consistency at this step proves crucial—the balance of sodium versus acetic acid tweaks flavor, shelf life, and microbial stability down the line.
Sodium diacetate doesn’t get too fancy in the lab. Its main move is acid dissociation—dump it in water, and acetic acid comes off, boosting tartness where it lands. When exposed to higher temperatures, much of the acetic acid drifts away as vapor, leaving sodium acetate behind. Food scientists sometimes explore tweaks—changing grain size or mixing with other flavor enhancers—to sharpen its performance in spice blends or seasoning packets. As a buffer, it helps stabilize foods, steering flavor away from spoilage and off-notes caused by pH swings. Under normal storage and handling, it won’t spring chemical surprises.
In the global food trade, sodium diacetate picks up a handful of nicknames and regulatory tags. E262(ii) flags its approval in the European Union. Some labels call it vinegar acid sodium salt or acid sodium acetate; others stick with its straightforward IUPAC name. Manufacturers selling to snack or bakery sectors usually keep it simple, listing “sodium diacetate” or “sour salt” right on the ingredient panel. Industry insiders might know it as CAS 126-96-5, a number that doesn’t trip off the tongue but tells chemical buyers exactly what’s in the sack.
Food safety authorities like the FDA, EFSA, and WHO keep tight control over food additives, including sodium diacetate. Toxicological studies and decades of consumption confirm its safety in the levels commonly found in processed foods. Plants that handle and package the material need well-lit, ventilated areas. Workers wear gloves and dust masks to steer clear of irritation. Good Manufacturing Practices (GMP) keep the risk of accidental mix-ups or contamination low. Temperature and humidity control matter during storage, too—fail at that, and the product can draw moisture or lose potency, affecting the taste of finished goods.
Most folks meet sodium diacetate on their favorite potato chips or tortilla crisps. Its punchy taste sparks up seasonings for popcorn, crackers, and salad croutons, and it’s a staple in commercial bread mixes to stop molds in their tracks. Beyond snack aisles, food processors add it to processed meats and cheeses for shelf life alone—nobody enjoys funky, slimy edges on a slice of ham. Outside the culinary world, swine and poultry feed producers count on it as a safeguard against bacterial spoilage, and it pops up in odor control products too. In my time working near bakery lines, every food technologist I met had a story about moldy bread suddenly staying fresh, all because someone added a pinch of sodium diacetate.
University labs and food R&D centers keep playing with sodium diacetate, chasing ways to improve food safety while dialing back synthetic chemicals. Researchers test how tweaks in acetic acid content affect preservation and flavor for gluten-free or low-carb breads. Others look at how blending it with natural spices or essential oils might suppress pathogens without overpowering taste. I’ve seen pilot studies showing that even microencapsulated forms can slow mold on pita chips or wraps, opening new directions for product innovation. Regulatory changes, like clean label initiatives, fuel more research into how sodium diacetate can work in natural and organic food lines.
Decades of studies run by government and independent labs point to a solid safety record for sodium diacetate at low concentrations. The EFSA established an acceptable daily intake—meaning you’d have to eat mountains of food loaded with it to hit anything close to risky levels. Short-term exposure mainly risks mild nose or eye irritation during handling in factories, so personal protection makes sense. Rats, dogs, and even humans in controlled studies didn’t see toxicity problems at amounts far above what’s eaten in a typical diet. Food safety panels keep reviewing fresh data, guarding against surprises, but so far the science lines up behind its safety in food.
With growing demand for clean labels and less synthetic-sounding additives, sodium diacetate stands as a bridge. It’s already backboned much of the snack and bakery industry, but new plant-based foods, next-gen protein bars, and minimally-processed snacks all need help to fight off spoilage and off-flavors. Companies keep looking for ways to pair sodium diacetate with other natural extracts or enzymes, aiming for shelf life without sacrificing consumer trust. Tech advances in particle size and microencapsulation could soon make it easier to use in gluten-free, allergen-friendly, and artisan-style products. Regulatory environments keep shifting, yet sodium diacetate’s long record and genuine origin in vinegar chemistry give it staying power where food makers and shoppers both want safe, straightforward ingredient stories.
Many people grab a bag of chips or a handful of beef jerky without thinking twice about what keeps that tangy bite alive or why the snack doesn’t spoil so quickly. Sodium diacetate steps in right here, quietly doing its job. This salt, made from blending sodium acetate with acetic acid, brings both flavor and shelf-life to the table. Walk through any grocery aisle and you bump into sodium diacetate more often than most folks realize.
Anyone who has tasted salt and vinegar chips has sodium diacetate to thank for that signature punch. This substance hits two birds with one stone: it brings saltiness and a vinegar-like sourness, but without soaking your snack. Companies lean on it because powders mix more evenly onto snacks than liquid vinegar. In my own experience interviewing snack manufacturers, many choose sodium diacetate for the intensity it adds without any mess.
Its use doesn’t end with chips. Pretzels, crackers, and roasted nuts often rely on sodium diacetate to reinforce or round out flavor. The consistent taste profile builds trust with customers. Fluctuating flavors lose repeat buyers, and business pays attention to that risk.
Preservation matters, especially for products with long journeys from factory to shelf. Sodium diacetate acts as a shield against mold and certain bacteria. In processed foods, where moisture and warmth hang around, contamination finds opportunity. Sodium diacetate changes the environment just enough to make life tough for spoilage microbes.
Food safety experts like the USDA flag sodium diacetate as a useful hurdle. Databases from groups like the FDA and European Food Safety Authority show that it maintains a strong track record for safety at approved levels. I’ve spoken to small-batch jerky makers who count on sodium diacetate as part of their food safety toolkit—especially those shipping across the country, where product might not be refrigerated every step of the way.
Industrial uses fly under the radar. Some in farming use sodium diacetate to manage mold in animal feed. Others in the cleaning industry like its ability to balance pH and curb unwanted smells in cleaning solutions. It shows up in water treatment, too. My time consulting in agriculture exposed how subtle tweaks to animal feed or silage can stop big losses to spoilage over time, and sodium diacetate played a distinct role there.
No food ingredient comes without debate. Some consumers worry about chemicals they can’t pronounce, even those the FDA recognizes as safe. Skepticism hooks into a bigger discussion around transparency in labeling. As someone who keeps a close eye on food labeling laws and changing market trends, I see brands moving to explain why sodium diacetate is there—not just burying it in a long list of ingredients. Being upfront wins trust.
Alternatives exist, though not always as effective or cost-friendly. Vinegar can do the same job but brings moisture and changes texture. Natural acids work as substitutes, but some stumble on the flavor front. Research continues on plant-based or fermented additives that could match what sodium diacetate offers, especially as shoppers push for “cleaner” labels.
Sodium diacetate stays popular because it delivers on several fronts: taste, safety, cost. How companies talk about it and how scientists continue to find new options both matter as shoppers care more about what they put into their baskets.
Sodium diacetate turns up on ingredient labels for potato chips, tortillas, and all kinds of snacks that line grocery shelves. It sounds complicated, but this ingredient comes from vinegar and sodium acetate and lands in food for a few main reasons: preserving freshness, adding a hint of tang, and keeping bacteria at bay.
Food safety stirs concern, especially when ingredients show up in lots of processed items. I remember the first time scanning an ingredients list for something unpronounceable and wondering, “Should this really go in my kid’s lunch?” That’s where trust in science helps bridge the gap.
The U.S. Food and Drug Administration lists sodium diacetate as GRAS, or “generally recognized as safe.” That didn’t happen overnight — it reflects decades of toxicology studies, peer review, and global food-safety organizations echoing similar findings. The Joint FAO/WHO Expert Committee on Food Additives sets acceptable daily amounts far above what most people would ever come close to eating.
In ordinary portions, sodium diacetate breaks down in the body in the same way as vinegar. It doesn’t gather inside organs. Researchers looked at people consuming much higher levels through food and didn’t find health issues tied directly to the ingredient. It helps suppress dangerous microbes, keeping prepared foods safe, which probably explains its appearance on so many labels.
For anyone with concerns about extra sodium, sodium diacetate brings in only a small amount compared to table salt. Still, lots of packaged snacks together add up. High sodium in any form poses a problem for people struggling with blood pressure. Anyone worried about sodium intake often benefits from looking at the total amount in all foods eaten, not just focusing on individual additives like this one.
Some people stay wary of ingredients that sound synthetic — maybe from memories of learning about food dyes or chemical preservatives that drew negative headlines years ago. It feels wise to pay attention and ask questions. I’ve had friends cut out processed foods for peace of mind, and that approach definitely limits exposure to all sorts of additives, including this one.
Restaurants and food companies keep using sodium diacetate because it reliably keeps food safe on shelves and maintains taste. For anyone looking to avoid additives, preparing food at home with simple ingredients works. Swapping packaged snacks for fruit, nuts, or plain yogurt cuts out sodium diacetate and most other additives in one step.
Staying informed about widely used ingredients pays off. Reading up from reliable sources like FDA, Mayo Clinic, and registered dietitians brings some peace of mind. Most evidence points to sodium diacetate as a safe ingredient when used within current guidelines. Any health concerns linked to excessive sodium probably matter more than the presence of sodium diacetate itself.
I always come back to the same principle: the fewer processed foods on the weekly menu, the less worry there is about hidden additives. For those not ready to swear off convenience foods, moderation and label reading make a real difference.
Walk through any supermarket, and you'll spot bags of bread, snacks, and deli meats claiming to stay fresh for days. For businesses, food waste quickly cuts into profit and reaches the consumer’s wallet as well. Sodium diacetate steps into this picture as a preservative that makes food last a bit longer. Its ability to control mold and bacteria means bread doesn't get that fuzzy coat nearly as quickly, and deli meats keep their appetizing look and flavor. Fewer spoiled products reduce loss for retailers and help ensure families aren’t tossing out food as often.
Pick up a package of flavored chips and the punch of tangy, savory taste comes through immediately. Sodium diacetate delivers not only preservation but also a distinct, piquant impact in foods ranging from snacks to sauces. It brings that signature vinegar snap without sogginess. For manufacturers searching for a reliable way to boost flavor and maintain a consistent taste, sodium diacetate serves as a real workhorse. I've worked in kitchens where the balance between salty and acidic flavors is tough to get right, and this ingredient gives food producers a tool to ensure every batch hits the same mark.
There’s a growing concern about what goes into processed food. Companies, by law, need to meet food safety standards. Sodium diacetate received approval from food safety authorities including the FDA and the European Food Safety Authority after extensive testing. This makes it a solution trusted by food producers, especially those supplying schools, care homes, and hospitals. Its safety record and effectiveness in small amounts bring peace of mind — offering an answer to microbial risks without adding unfamiliar or harsh chemicals. That type of assurance matters in facilities where controlling pathogens makes all the difference.
Margins stay tight across the food business. Adding a preservative that does double duty — extending shelf life and enhancing taste — means smaller businesses avoid losses from spoilage and can send better-tasting products out with confidence. Even large-scale producers value sodium diacetate for its predictability and the ease with which it fits into standard production lines. The costs of ingredient loss, recalls, or flavor failures can be devastating. Reliable ingredients help keep those risks in check.
More people read labels now than ever before. Shoppers ask direct questions about what is in their food, how it’s made, and the reasons behind each ingredient. Sodium diacetate, often labeled simply as “acidity regulator” or “preservative,” is straightforward, and regulatory guidance means its use is well-understood. In my own circles, parents and cooks alike appreciate clarity, and the short list of roles sodium diacetate plays fits into that expectation.
Food science doesn’t need to make food feel synthetic or foreign. Used well, sodium diacetate provides real advantages — fights spoilage, bolsters taste, helps businesses save money, and reassures buyers who look for recognizable ingredients. Better shelf life, reliable flavor, and a clean profile aren't just company goals; they matter to families, schools, and local shops trying to bring dependable options to the table.
Walk into any grocery store and scan the ingredient lists on bread, snacks, or ready-to-eat meals, and you might notice two familiar names: sodium diacetate and acetic acid. They both show up in foods for a reason—both tie back to vinegar and sourness—but they don’t work the same way or taste quite the same.
Acetic acid goes by another name most of us know: the main compound that gives vinegar its trademark bite. A splash of white vinegar brightens a salad, makes pickles sharp, or even helps unclog a drain. In food, acetic acid drops the pH, which keeps some bacteria at bay and makes a product last longer.
Sodium diacetate, on the other hand, comes from a mix of vinegar’s acetic acid and sodium acetate—a salt made for food use. It usually shows up as a dry, powdery ingredient in foods where a bitter vinegar taste may not work. Bakers reach for sodium diacetate to keep mold away from sliced bread or tortillas without soaking the food in sourness.
Anyone who’s made a batch of pickles or marinated cucumbers knows acetic acid packs a punch. Its liquid form makes it easy to blend into dressings and sauces, but it also carries a strong aroma. Too much, and you end up with something that smells like a cleaning supply aisle.
In my own kitchen and in bakeries I’ve toured, sodium diacetate wins points for subtlety. Tossing this dry powder into flour brings a touch of tartness, not a wallop of vinegar. It takes care of mold, helps slow some bacteria, and doesn’t water down or overpower delicate flavors. Tortilla factories, for example, use sodium diacetate to keep flatbread soft and fresh—but without the wafting scent of vinegar that would turn off most shoppers.
Both sodium diacetate and acetic acid carry approval from food safety authorities like the FDA. They’ve each been tested for decades. Still, there’s a difference in how much or how safely you can use them. Pure acetic acid—at high strengths—turns from food friendly to hazardous. In many commercial kitchens, staff wear gloves and eye protection to avoid burns from liquid acetic acid.
Sodium diacetate avoids a lot of this risk. Its powder form travels better, blends into bulk mixes without special gear, and steers clear of the intense, eye-stinging vapors. Less risk means fewer accidents, less spill clean-up, and a more comfortable work space for people in food production.
Back when I worked in a small sandwich shop, we fought weekly against moldy bread in summer. Adding straight vinegar would just ruin the taste, so instead, we looked up how bigger bakeries solved it. Sodium diacetate became the answer—it stretched shelf life and left no strong aftertaste.
For those working in food production, or even home cooks baking in humid climates, understanding the right ingredient makes a difference. Sodium diacetate has its place: subtle, easy to handle, and built for modern recipes that depend on both taste and safety. Acetic acid’s sharper edge belongs where punch and brightness matter. Both ingredients offer value—but knowing the way they behave in practice changes what ends up on your table.
Sodium diacetate often pops up in seasonings and food preservation, especially in snack foods, meat products, and baking mixes. Its place in food and other everyday products makes proper storage a matter worth attention. If you ever grabbed a container of salt and found clumps, you’ve already experienced one of the biggest risks: moisture. This is even more important with sodium diacetate, since it pulls in water from the air even faster than table salt. Leave it exposed, and it hardens quickly or dissolves partially, affecting both its use and safety.
Sodium diacetate, a double salt made from sodium acetate and acetic acid, looks like a white, free-flowing powder when stored right. This appearance signals it won’t clog up equipment in food processing plants or ruin recipes in your kitchen. The moment it picks up excess moisture, though, it starts to cake and lose its performance as a preservative and flavoring. According to the United States Pharmacopeia and food ingredient suppliers, any open container will quickly attract enough water from the air to spoil the product. Moisture also increases the risk of chemical breakdown, leading to off-odors or changes in potency. So, keeping this powder dry keeps it safe, stable, and effective.
In my years working with food ingredients, I’ve seen the simplest rules work best. Store sodium diacetate in a cool, dry storeroom, away from sources of heat, steam, and direct sunlight. Rooms with an air conditioner or dehumidifier usually fare much better because fluctuations in humidity make the powder clump. Big drums and smaller pails must stay tightly sealed. Even a few minutes with a loose lid, especially on humid days, sets the stage for wasted product.
Keep the original packaging intact as long as possible. Most commercial sodium diacetate comes packed in polyethylene-lined kraft bags or food-grade drums with solid closures. Transferring to glass or rigid plastic containers with moisture-proof seals at home also works. Just don’t trust thin zip bags or open bowls—the powder will attract smells and moisture from the air. Any employee in a food plant or home cook can reduce risk by wearing clean gloves and never scooping out product with a wet utensil. Cross-contamination, even on a small scale, shortens shelf life and invites unexpected quality issues.
Improper storage not only reduces the shelf life but can also increase safety hazards. Absorbed water allows bacteria or molds to thrive, especially if the powder winds up stuck to the sides of a damp container for a long period. This spoils the food it’s meant to preserve and puts people at risk. The Occupational Safety and Health Administration recommends labeling all storage bins clearly, keeping them away from incompatible chemicals like strong oxidizers, and setting up a first-in, first-out approach to ensure nothing sits around too long. These steps matter in workplaces and commercial kitchens, where larger quantities raise the stakes.
Humidity jumps up in summer and in coastal areas, so storage strategies shift with the seasons. Many businesses install silica gel packets or dedicated moisture absorbers in storerooms. It’s a simple step but makes a noticeable difference. In smaller kitchens, keeping small batches in sealed jars and quickly resealing after each use stops spoilage. Training employees and home users alike to spot changes—like lumpiness or sour smells—helps catch issues before they cause bigger problems.
Treat sodium diacetate like quality baking powder: dry, cool, sealed, and labeled. Small steps save money, cut waste, and make the food on our tables safer.
| Names | |
| Preferred IUPAC name | Sodium acetate ethanolate |
| Other names |
Sodium hydrogen diacetate
Sodium acid acetate Diacetic acid, sodium salt |
| Pronunciation | /ˌsəʊdiəm daɪˈæsɪteɪt/ |
| Identifiers | |
| CAS Number | 126-96-5 |
| 3D model (JSmol) | `[Na+].CC(=O)[O-]` |
| Beilstein Reference | 1738734 |
| ChEBI | CHEBI:32944 |
| ChEMBL | CHEMBL1371 |
| ChemSpider | 31951 |
| DrugBank | DB11110 |
| ECHA InfoCard | 100.028.427 |
| EC Number | 262-013-3 |
| Gmelin Reference | 5293 |
| KEGG | C14428 |
| MeSH | D016725 |
| PubChem CID | 23665763 |
| RTECS number | AJ4300010 |
| UNII | SP542X5X4D |
| UN number | UN2341 |
| Properties | |
| Chemical formula | NaC₂H₃O₂·CH₃COOH |
| Molar mass | 142.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Acetic acid-like |
| Density | 1.528 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.62 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.75 |
| Basicity (pKb) | 4.75 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.394 |
| Viscosity | Water = 50 mPa·s (25°C, 30% aq. sol.) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 198.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -722.0 kJ/mol |
| Pharmacology | |
| ATC code | A01AB58 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, Exclamation mark |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a dry place. Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. Wear protective gloves/eye protection/face protection. |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: - |
| Autoignition temperature | 250 °C |
| Lethal dose or concentration | LD50 (oral, rat): 4,393 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4970 mg/kg (rat, oral) |
| NIOSH | NA2346 |
| REL (Recommended) | 20 mg/kg bw |
| Related compounds | |
| Related compounds |
Sodium acetate
Acetic acid Calcium acetate Potassium acetate Magnesium diacetate |